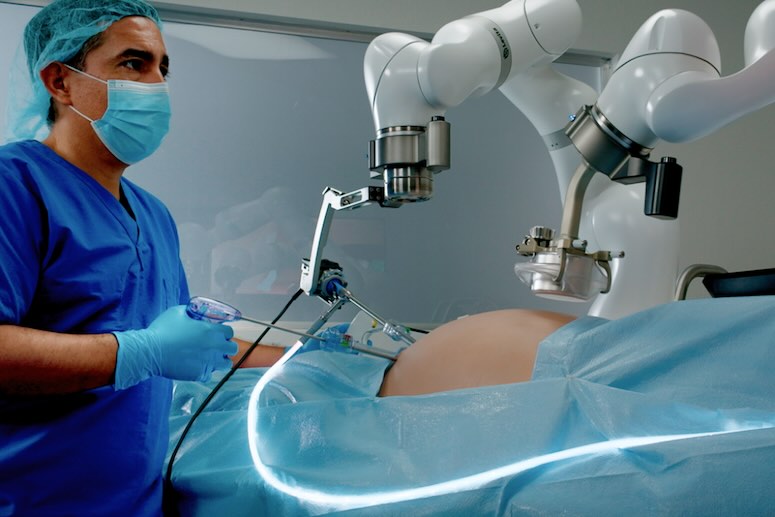
Levita Magnetics this week announced it received expanded FDA 510(k) clearance for its MARS (magnetic-assisted robotic surgery) system.
The FDA granted 510(k) clearance for its Magnetic Surgical System (MSS), combined with MARS, in bariatric and hiatal hernia repair. Mountain View, California-based Levita said the expanded indication reinforces its ability to support surgeons treating patients with obesity-related conditions. It provides a less invasive solution that enables simultaneous treatment of hiatal hernias during bariatric surgery.
FDA clearance enables clinicians to use the MARS system’s Dynamic Magnetic Positioning technology. This retracts the liver and enhances visibility and access during hiatal hernia repairs. Clearance offers an innovative, less invasive option for abdominal surgery and paves the way for broader adoption of the technology, Levita said.
“This milestone reinforces our commitment to expanding access to better surgery,” said Dr. Alberto Rodriguez-Navarro, founder and CEO of Levita Magnetics. “We’re building a future where less invasive means more effective.”
Additionally, the FDA gave the green light to the company’s new 12.5 mm magnetic grasper. Levita engineered the grasper to improve visualization and control in patients with high BMI and large or difficult-to-retract livers. The device allows surgeons to grasp the liver more centrally, rather than from the edge. This improves surgical access in both hernia and bariatric procedures.
MARS introduces a new paradigm of robotic surgery, using magnetic technology to reposition organs internally. It reduces the number of incisions and enables complete control during laparoscopic procedures.

Levita Magnetics’ MARS system uses magnetic forces to control a grasper instrument inside a patient. | Credit: Levita Magnetics
The system comes in a compact footprint designed to fit into existing operating rooms. It builds on the success of the company’s first commercial product, the Levita Magnetic Surgical System. Levita designed MARS to deliver the same patient benefits while empowering surgeons with increased control of surgical instruments.
Levita Magnetics won a 2025 RBR50 Robotics Innovation Award for a dual-robot surgery that successfully removed a human prostate. The procedure used the MARS platform to maneuver internal organs. Dr. Jeffrey Cadeddu at the University of Texas Southwestern Medical Center combined the proprietary magnetic positioning system with Intuitive Surgical‘s Da Vinci SP (single-port) robot. During the procedure, the surgical team used the MARS platform to retract tissue within the patient precisely. This increases visualization, allowing the team to preserve nerves.
The Da Vinci SP provided the capabilities required for this complex urological surgery. The Intuitive Surgical robot makes an incision so that surgeons can get inside the patient and perform a procedure. The MARS system allows the surgeons greater access and visualization once they’re inside the body, removing the limits that come with single-port surgery.
Editor’s Note: This article was republished from sister website MassDevice.
The post Levita Magnetics MARS surgical robot receives expanded FDA clearance appeared first on The Robot Report.